2nd Generation of Liver Cancer Early Screening-MPM
Products and Services
- Establishment and Consulting of LDTs LabEpiSante Biomedical - Establishment and Consulting of LDTs Lab | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- Reagents and KitsEpiSante Biomedical - Reagents and Kits | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- Customized ServiceEpiSante Biomedical - Customized Service | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
MPM - 2nd Generation of Liver Cancer Early Screening
The second-generation ctDNA methylation liver cancer early screening testing is an innovative non-invasive blood testing technology that can diagnose the early stage of liver cancer more accurately. MPM covers more methylation sites, which not only increases the stability of the test results, but also improves the sensitivity of the early detection.
Non-invasive
Blood testing, non-ivasive
Dynamic
DNA methylation is dynamic and can be monitored
Accurate
Both sensitivity and specificity are superior to the current cancer markers.
Early Diagnostic
detecting at an early stage of liver cancer
The Faults of the Current Liver Cancer Testing
AFP (Alpha-fetoprotein)
A serum protein biomarker, which is the gold standard for liver cancer screening. But its performance is not satisfactory, and the sensitivity is only 10-20% for the detection of early liver cancer. Moreover, about 30% of patients will not have elevated AFP levels even at the end stage of liver cancer.1。
PIVKA-II (Protein induced by Vitamin K absence or antagonists-II)
Another serum protein biomarker, which has a sensitivity of about 34-40% for the early detection of liver cancer 2。
Abdominal Ultrasound
An imaging test, which sensitivity is related to the instrument equipment, operator experience, tumor size, location, shape, etc., and has a sensitivity of about 25-65% for the early screening of liver cancer 3。
1. Clinica Chimica Acta 2008; 395: 19-26.
2. Cancer Epidemiol Biomarkers Prev. 2020; 29:2495-2503.
3. Gastroenterology 2018; 154: 1706-1718.
A serum protein biomarker, which is the gold standard for liver cancer screening. But its performance is not satisfactory, and the sensitivity is only 10-20% for the detection of early liver cancer. Moreover, about 30% of patients will not have elevated AFP levels even at the end stage of liver cancer.1。
PIVKA-II (Protein induced by Vitamin K absence or antagonists-II)
Another serum protein biomarker, which has a sensitivity of about 34-40% for the early detection of liver cancer 2。
Abdominal Ultrasound
An imaging test, which sensitivity is related to the instrument equipment, operator experience, tumor size, location, shape, etc., and has a sensitivity of about 25-65% for the early screening of liver cancer 3。
1. Clinica Chimica Acta 2008; 395: 19-26.
2. Cancer Epidemiol Biomarkers Prev. 2020; 29:2495-2503.
3. Gastroenterology 2018; 154: 1706-1718.
Our Advantages
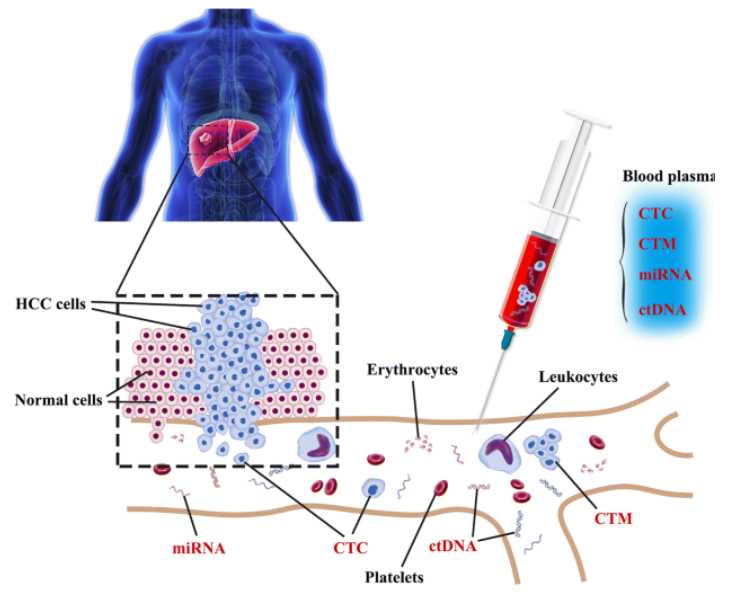
MPM detects methylation markers on the circulating tumor DNA (ctDNA), released from cancer cells into the blood. These methylation markers have very high sensitivity and specificity, especially in the early stages of liver cancer. MPM has a diagnostic sensitivity as high as 90%, and it can be used in combination with current blood tests to compensate for the insufficient sensitivity of AFP or PIVKA-II.
When the results of imaging examinations (such as abdominal ultrasound, CT scan, etc.) are unclear, MPM can assist doctors in making better assessments.
When the results of imaging examinations (such as abdominal ultrasound, CT scan, etc.) are unclear, MPM can assist doctors in making better assessments.
MPM Technology
Who needs the testing?
It is recommended that the high-risk population undergo testing every six months to a year.
- Hepatitis B/C virus carriers
- Patients with cirrhosis
- Patients with chronic hepatitis
- Having a family history of liver cancer
- Long-term smokers and drinkers
- People with obesity and fatty liver
- Imaging examination is unclear
Clinical Performance of MPM
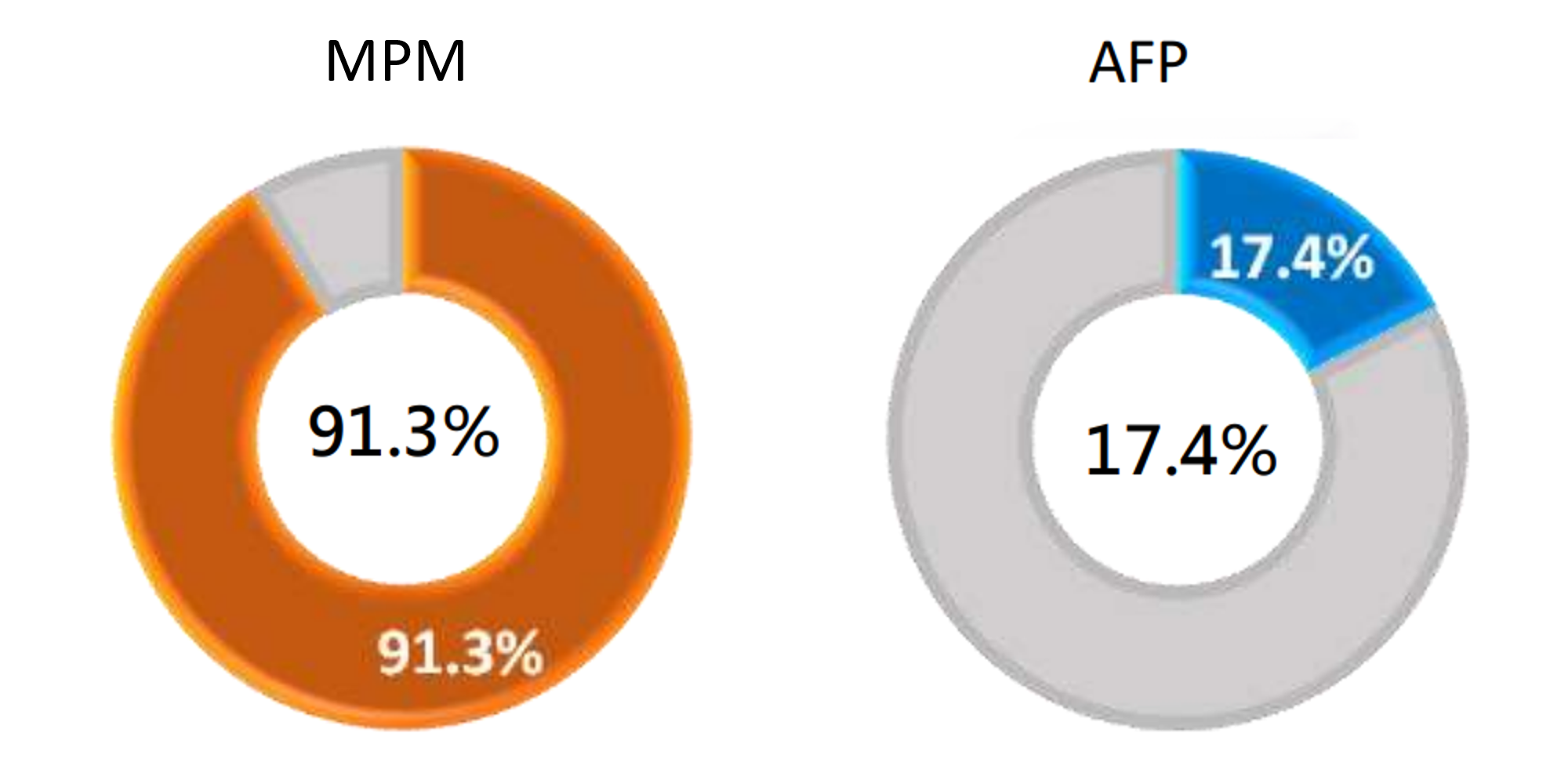
Clinical Performance of MPM - Early Detection
Clinical Performance of MPM - AUC
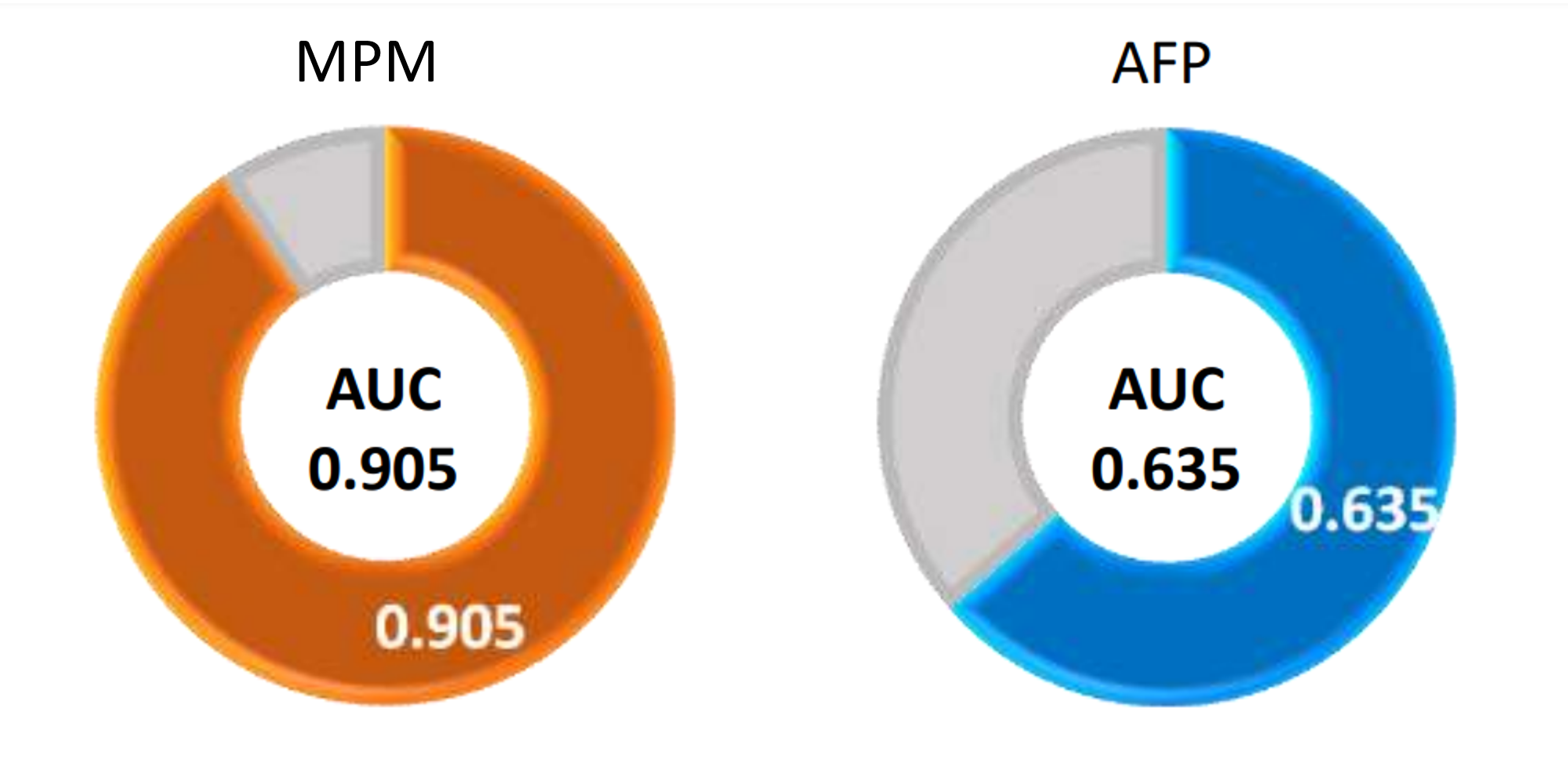
AUC (Area Under Curve) is the area under the ROC curve, representing the ability to predict cancer. AUC=1 means both sensitivity and specificity are 100%. AUC = 0.5 represents no discrimination; AUC between 0.6 and 0.7 is acceptable discrimination; AUC between 0.7 and 0.8 has excellent discrimination; AUC between 0.9 and 1 represents excellent discrimination.
Clinical Performance of MPM - Sensitivity
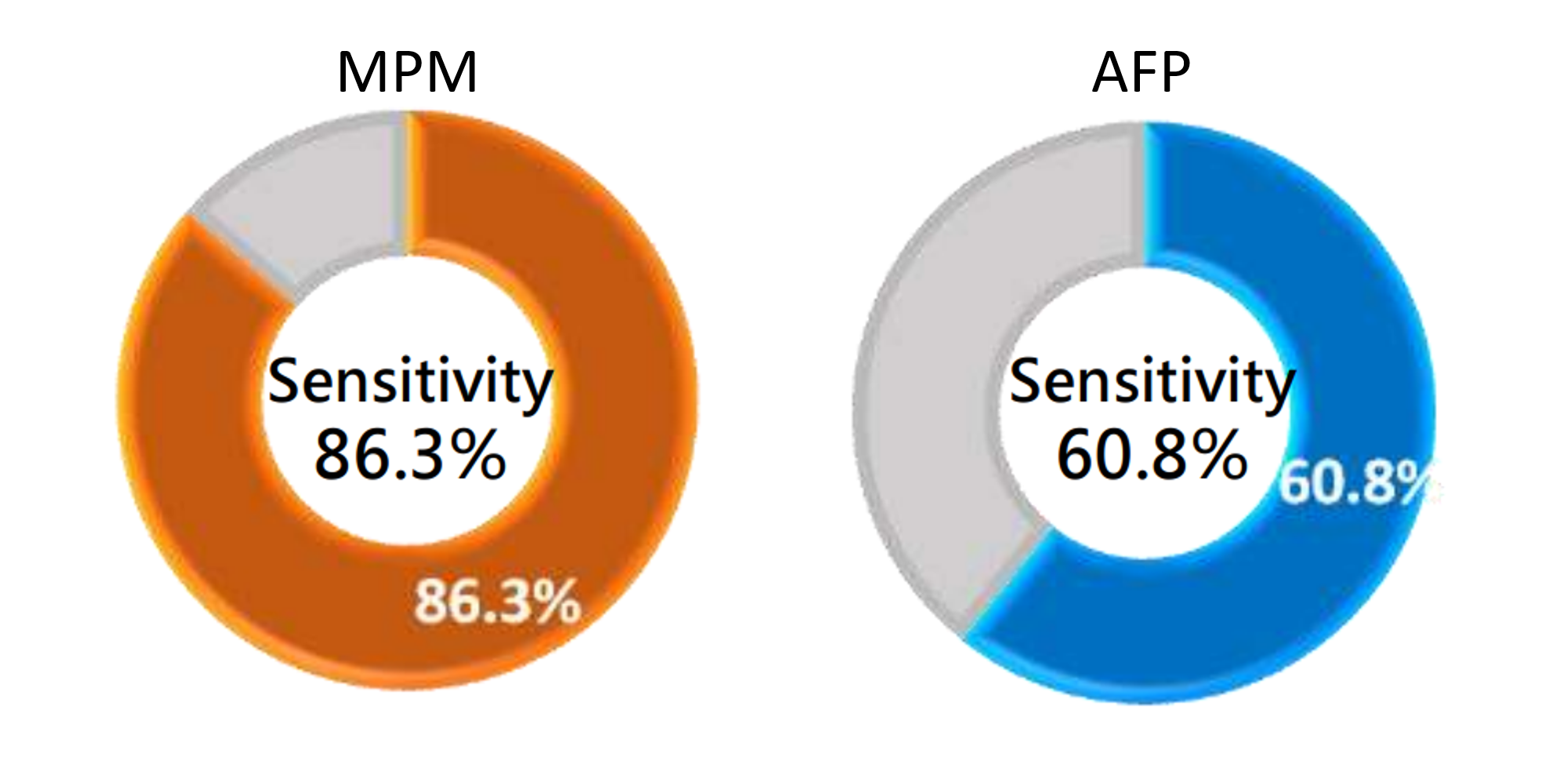
Sensitivity, also known as the true positive rate, refers to the proportion of actual liver cancer samples that are detected and diagnosed as liver cancer.
Clinical Performance of MPM - Specificity
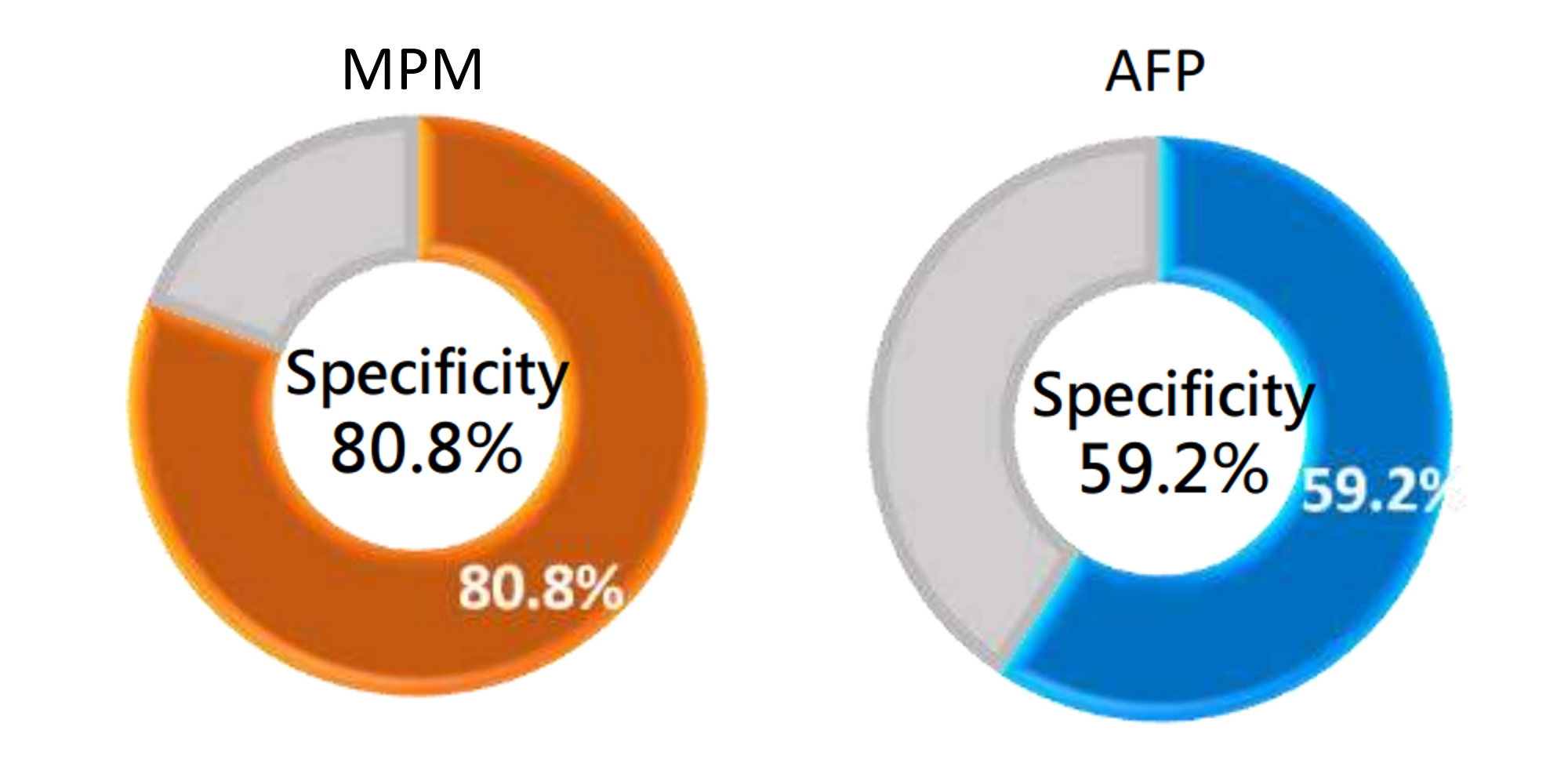
Specificity, also known as the true negative rate, refers to the proportion of actual non-liver cancer samples that are pedicted to be non-liver cancer.