EpiSante Biomedical | LiverAM-Prognosis and Monitoring of Liver Cancer Ablation | DNA methylation analysis | cancer management
Products and Services
- Testing Service
- 2nd Generation of Liver Cancer Early Screening-MPMEpiSante Biomedical - 2nd Generation of Liver Cancer Early Screening-LiverES | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- Prognosis of liver Cancer Resection-LiverRPEpiSante Biomedical - Prognosis of liver Cancer Resection-LiverRP | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- CancerMeth-Cancer Gene Methylation TestEpiSante Biomedical - CancerMeth-Cancer Gene Methylation Test | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- LiverMeth-Liver Cancer Gene Methylation TestEpiSante Biomedical - LiverMeth-Liver Cancer Gene Methylation Test | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- Global DNA Methylation TestEpiSante Biomedical - Global DNA Methylation Test | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- MethClock-Biological Age Anti-aging TestEpiSante Biomedical - Global DNA Methylation Test | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- Establishment and Consulting of LDTs LabEpiSante Biomedical - Establishment and Consulting of LDTs Lab | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- Reagents and KitsEpiSante Biomedical - Reagents and Kits | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
- Customized ServiceEpiSante Biomedical - Customized Service | DNA methylation | methylation, cancer testing, liver cancer, liver testing, liquid biopsy, liver prognosis, liver ablation, liver resection, immunotherapy, cell therapy
LiverAM-Prognosis and Monitoring of Liver Cancer Ablation
Ablation is a non-invasive, low-risk, low-cost, and effective treatment, so it has been widely used in recent years, especially for early-stage liver cancer patients who cannot undergo liver resection or transplantation. The five-year survival rate of early-stage liver cancer patients treated with ablation is comparable to that of resection surgery. However, within five years after ablation, there is still a high recurrence rate of 60%-85%. Prognosis and monitoring after ablation are crucial to the survival rate of liver cancer patients.
The current liver cancer markers that can be monitored are only serum protein AFP or PIVKAII. However, these two serum markers not only have low sensitivity, but also do not show a high proportion in early-stage liver cancer patients, which makes monitoring challange.
LiverAM can serve as an indicator for ablation prognosis and monitoring, supplementing the poor performance of AFP or PIVKA-II. It can also timely evaluate the treatment effect after ablation, thereby improving the survival rate of patients.
The current liver cancer markers that can be monitored are only serum protein AFP or PIVKAII. However, these two serum markers not only have low sensitivity, but also do not show a high proportion in early-stage liver cancer patients, which makes monitoring challange.
LiverAM can serve as an indicator for ablation prognosis and monitoring, supplementing the poor performance of AFP or PIVKA-II. It can also timely evaluate the treatment effect after ablation, thereby improving the survival rate of patients.
Our Advantages
World-leading
LiverAM is the world's first product that uses methylation markers for the prognosis and monitoring of liver cancer ablation.
Precise
Compared to the current biochemical markers and clinical pathological evaluations, LiverAM can serve as a more precise assessment tool for ablation prognosis and monitoring.
Convenient
LiverAM is a test for ctDNA in the blood, which is a type of liquid biopsy. It is noninvasive, safe, and convenient for monitoring.
Innovative
LiverAM uses qMSP technology, combined with an exclusive patented ablation methylation panel, to improve precision and detectionthroughput.
Who needs the testing?
Patients treated with ablation therapy
Clinical Performance of LiverAM
Clinical Performance of LiverAM - AUC
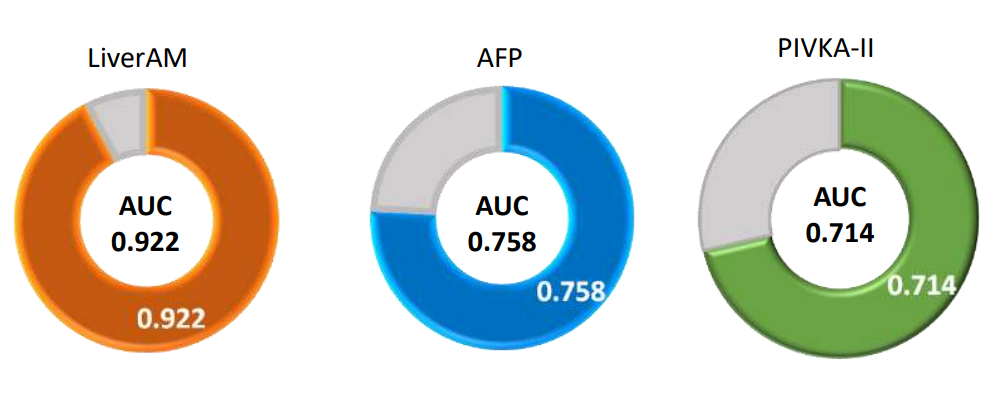
AUC (Area Under Curve) is the area under the ROC curve, representing the ability to predict cancer.
AUC=1 means both sensitivity and specificity are 100%. AUC = 0.5 represents no discrimination; AUC between 0.6 and 0.7 is acceptable discrimination; AUC between 0.7 and 0.8 has excellent discrimination; AUC between 0.9 and 1 represents excellent discrimination.
Clinical Performance of LiverAM - Sensitivity
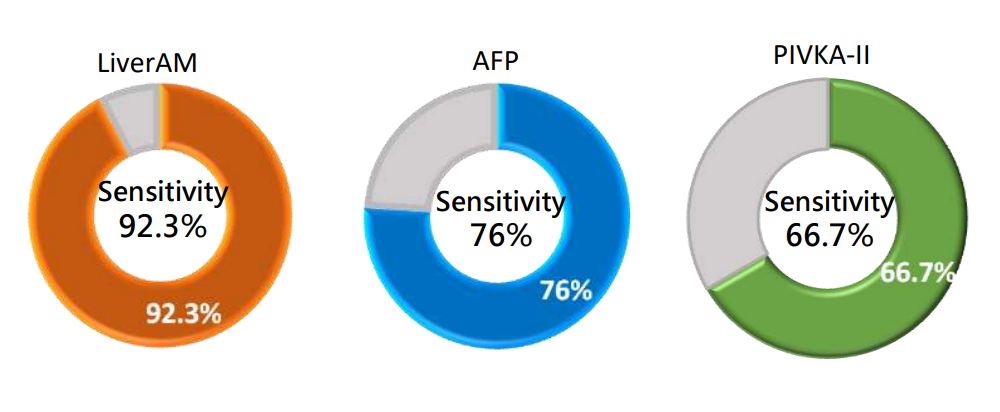
Sensitivity, also known as the true positive rate, refers to the proportion of samples that are actually effective for liver cancer ablation and are detected as effective for liver cancer ablation
Clinical Performance of LiverAM - Specificity
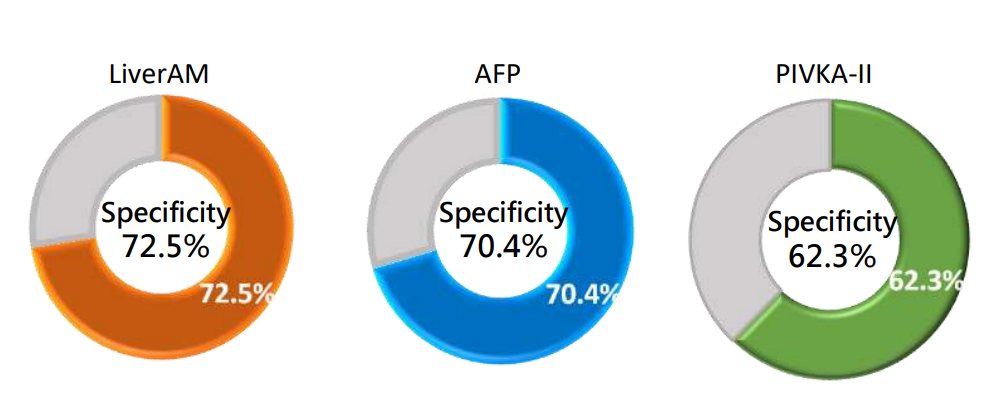
Specificity, also known as the true negative rate, refers to the proportion of samples that are actually not effective for liver cancer ablation and are determined to be not effective for liver cancer ablation
Clinical Performance of LiverAM - Monitoring
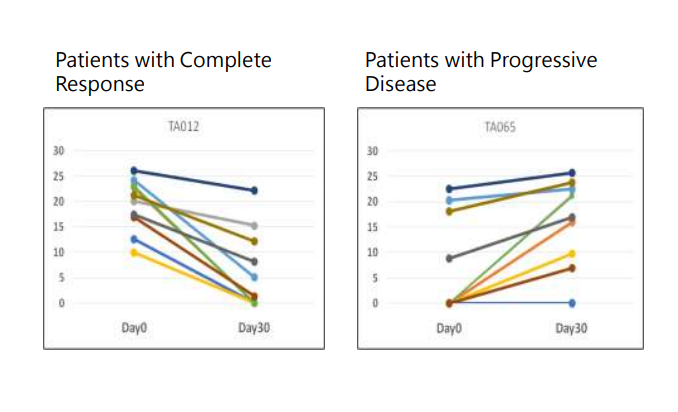
For patients who respond well to ablation, the methylation markers decrease after the surgery. For patients who do not respond well to the ablation, the methylation markers remain stable or increase after the surgery. There is a significant difference between the two, and the ablation prognosis monitoring panel can serve as a good predictive tool.
