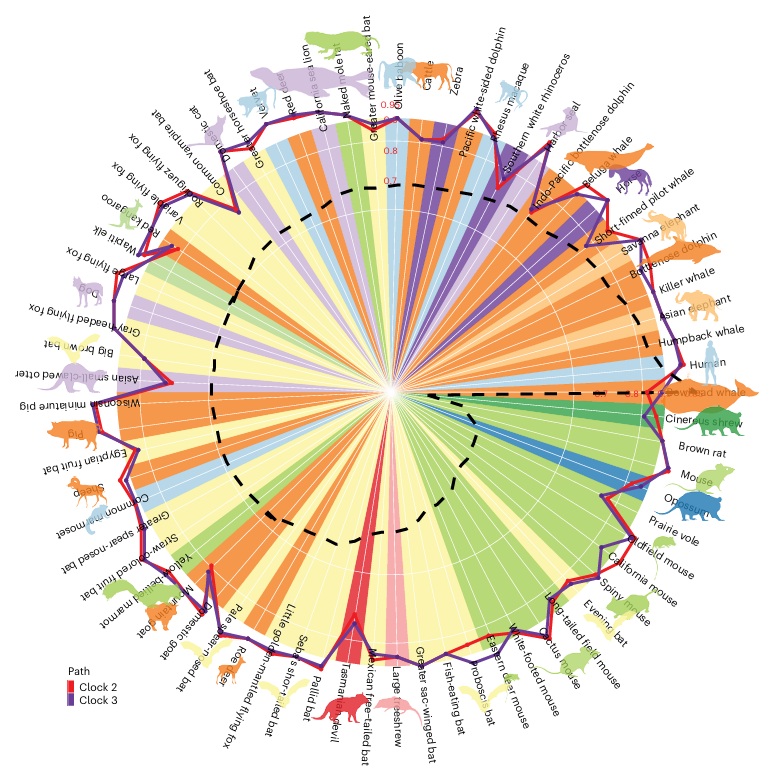
Aging, often considered a result of random cellular damage, can be accurately estimated using DNA methylation profiles, the foundation of pan-tissue epigenetic clocks. This study demonstrates the development of universal pan-mammalian clocks, using 11,754 methylation arrays from Mammalian Methylation Consortium. These predictive models estimate mammalian tissue age with high accuracy (r > 0.96). This study identified specific cytosines with methylation levels that change with age across numerous species. These sites, highly enriched in polycomb repressive complex 2-binding locations, are near genes implicated in mammalian development, cancer, obesity and longevity. The findings of this study offer new evidence suggesting that aging is evolutionarily conserved and intertwined with developmental processes across all mammals.
Aging is associated with multiple cellular changes that are often tis sue specific. Cytosine methylation, however, stands out, as it allows for the development of pan-tissue aging clocks (multivariate age estimators) that are applicable to all human tissues. The subsequent development of similar pan-tissue clocks for mice and other species suggests a conserved aspect to the aging process. For this purpose, this study employed the mammalian methylation array, which they recently developed to profile methylation levels of up to 36,000 CpG sites with flanking DNA sequences highly conserved across the mammalian class. This study employed such profiles from 11,754 samples from 59 tissue types, originating from 185 mammalian species with ages ranging from prenatal to 139 years old bowhead whale.
This study has certain limitations. The study primarily focuses on highly conserved DNA sequences, thus limiting their examination to approximately 36,000 CpG sites of the tens of millions that exist in most mammalian genomes. Additionally, their array platform exhibits a slight bias, featuring more probes that align with eutherian genomes than with marsupial genomes8. Overall, their results demonstrate that select epigenetic aging effects are universal across all mammalian species and capture multiple processes and manifestations of age that have thus far been thought to be unrelated to each other. This study expects that the availability of pan mammalian epigenetic clocks will open the path to uncovering interventions that modulate conserved aging processes in mammals.
Reference:
Lu, A.T., Fei, Z., Haghani, A. et al. Universal DNA methylation age across mammalian tissues. Nat Aging 3, 1144–1166 (2023).
08-22-2025 updated.